Venous protection in 2019: A year in review
Advancements in the management of patients with VTE
This year marked a significant milestone in the rapidly evolving therapeutic landscape for venous thromboembolism (VTE). In late August, the much-anticipated updated guidelines for the diagnosis and management of acute pulmonary embolism (PE) were presented at the European Society of Cardiology (ESC) congress.1 These updates show a strengthening of the recommendations around duration of therapy, the choice of dose, early discharge and home management, and the treatment of patients with cancer and acknowledge the robustness of a wealth of new data from clinical trials. In particular these guideline improvements are attributed to the strong clinical evidence from trials such as the pooled analysis of EINSTEIN EXT and EINSTEIN CHOICE,2 and the results of HoT-PE,3 select-d4 and Hokusai-VTE-Cancer.5 This progress could help to improve treatment and protect patients from potentially life-threatening VTE recurrence, providing them with a more certain future.

Key updates in the 2019 ESC guidelines for the treatment of acute PE1
Outpatient management of PE
The HoT-PE study found that early discharge and home treatment with rivaroxaban is effective and safe in carefully selected patients with acute PE at low risk of mortality (acute low-risk PE).3,6 Therefore, rivaroxaban may simplify the outpatient treatment of acute PE by providing a tailored dose according to the patient’s risk profile, without compromising on efficacy3,7In addition, reductions in hospital length of stay can have an impact on healthcare costs and improve patient satisfaction and quality of life.3,6
The 2019 ESC guidelines recommend that carefully selected patients with low-risk PE should be considered for early discharge, to allow these patients to be treated in the comfort of their own home, if proper outpatient care and anticoagulation can be provided (Class IIa, Level of evidence A).1 The level of evidence was B in the previous 2014 update.8
Cancer-associated thrombosis
The phase III Hokusai-VTE-Cancer study5 and the select-d pilot study4 provide the first randomized comparisons of NOACs versus low molecular weight heparin (LMWH) in patients with cancer-associated thrombosis (CAT).
In the Hokusai-VTE-Cancer study, treatment with edoxaban (60 mg once daily [od]) for up to 12 months was found to be non-inferior to dalteparin for the composite primary outcome of recurrent VTE or major bleeding in patients with CAT.5 The results of the select-d study demonstrated that rivaroxaban (15 mg twice daily [bid] for 3 weeks followed by 20 mg od) was associated with a lower cumulative rate of VTE recurrence at 6 months than dalteparin in patients with CAT.4 By replacing an injectable with an oral treatment tailored to their needs and risk profile, NOACs can reduce the burden on patients with CAT and allow them to focus on other challenges in their daily lives.4,5,9
Based on these data, NOACs may be an effective alternative to LMWH for the treatment of VTE in patients with cancer. However, the increased risk of bleeding, particularly in certain subsets of patients with cancer,4,5 suggests that patients who might benefit from this regimen need to be selected carefully.
For more information visit: https://www.thrombosisadviser.com/thrombosis-digest/2019/vte-cat-burden-injections
In September, patient-reported outcomes from the COSIMO study were presented at the European Society for Medical Oncology (ESMO) congress.10 The unique perspective gained from this study was of patients with CAT who had sequentially experienced both traditional anticoagulation (parenteral therapy or a vitamin K antagonist [VKA]) and rivaroxaban therapy after opting for this change.10 Overall, the patient-reported experience was a durable improvement in anticoagulation-associated treatment satisfaction, specifically a reduction in the perceived burdens of therapy, following the change from a LMWH or VKA to rivaroxaban.10 These data could mean improved adherence to, or persistence with, therapy for optimal, tailored protection against recurrent VTE, and indicate that patients with CAT – who may already face a gruelling treatment schedule – can rely on an anticoagulant therapy that allows them to focus on other challenges in their daily lives.
This year, a change in the CAT treatment paradigm was marked by major changes to key guidelines. For example, in the new ESC guidelines, rivaroxaban or edoxaban are now recommended as an alternative to LMWH, with advised caution for patients with gastrointestinal cancer.1
Selection of patients for extended treatment
A clinically important question is how best to select patients for extended anticoagulation in order to protect their future from recurrent VTE. Deciding on the optimal duration of anticoagulation therapy requires careful consideration of the risk of recurrence if treatment is stopped and the risk of bleeding if it is continued.1,15
We know that the risk of recurrence in patients with VTE provoked by major surgery is ~1–3% within 1 year after stopping anticoagulation.1,15 As such, treatment can be limited to 3 months in these patients.15,16 In contrast, in patients with unprovoked VTE, the risk of recurrent VTE after discontinuation of therapy is approximately 10% after 1 year, and 30% after 5 years.15 Therefore, extended treatment beyond the initial 3 months is recommended in such patients as long as their risk of bleeding is low.16 However, what about patients at clinical equipoise for extended treatment?
EINSTEIN CHOICE, a randomized, double-blind, phase III study, compared the efficacy and safety of two doses of rivaroxaban (20 mg od or 10 mg od) with aspirin in patients with VTE who had completed 6–12 months of anticoagulation therapy and were at equipoise regarding their need for continued anticoagulation.17 Rivaroxaban demonstrated superior efficacy to aspirin, at both doses, reducing the relative risk of recurrent VTE by approximately 70%.17 The incidence of major bleeding was less than 1% across the treatment arms17 Based on these results, the indicated dose of rivaroxaban for the extended prevention of recurrent VTE is rivaroxaban 10 mg od, unless the risk of recurrent VTE is considered high, such as in patients with complicated co-morbidities or those who have developed recurrent VTE on extended prevention with this dose.7
In a pooled analysis of EINSTEIN EXT and EINSTEIN CHOICE, the risk of VTE recurrence in patients with VTE provoked by minor persistent or transient risk factors was reported to be similar to that of patients with unprovoked VTE, suggesting that these patients may also benefit from extended anticoagulation.2 The analysis also showed that rivaroxaban is an effective option for extended treatment regardless of risk factor profile.2
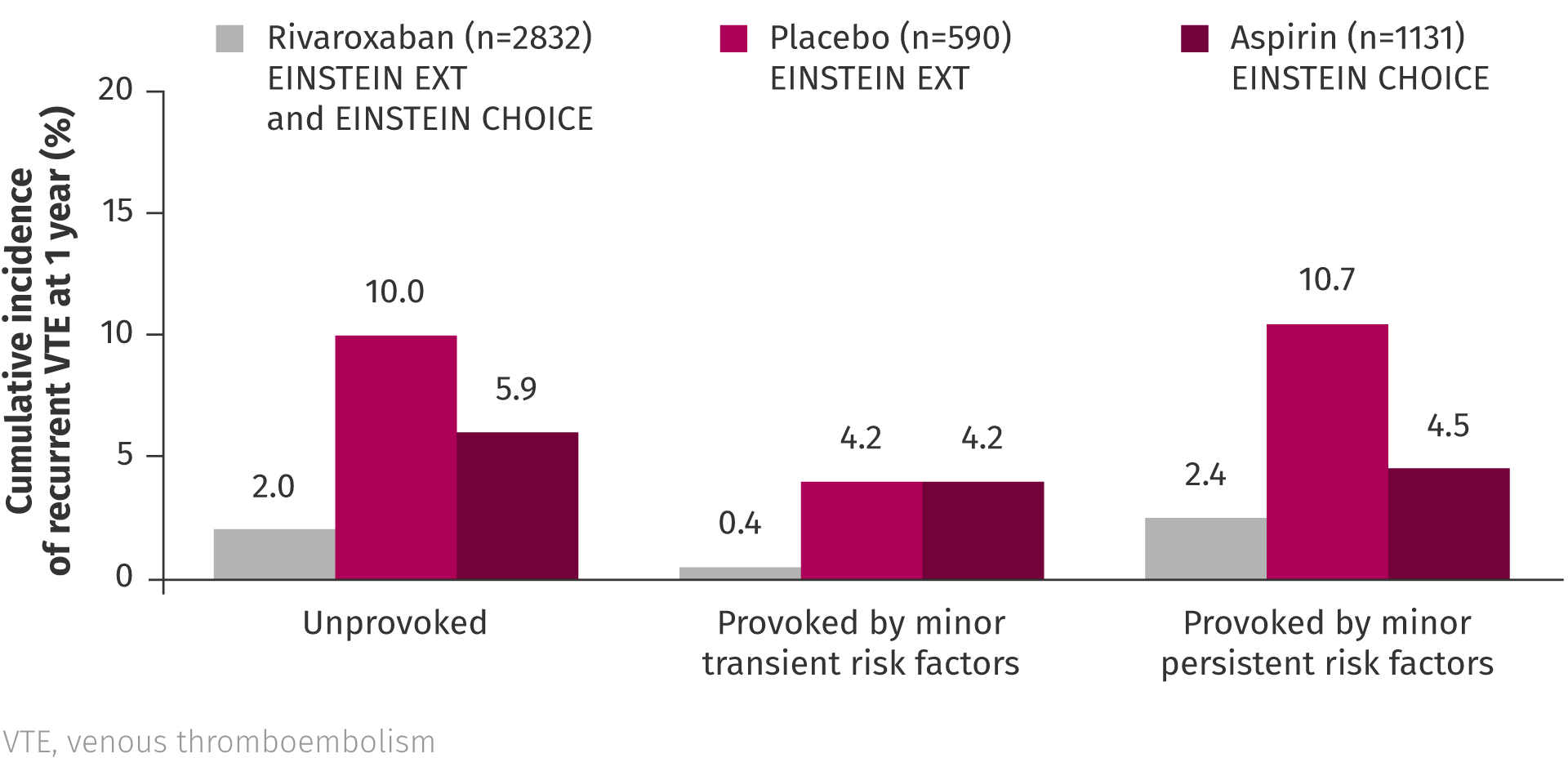
Pooled analysis of EINSTEIN EXT and EINSTEIN CHOICE2
Summary
The year 2019 has seen exciting new data, along with guideline updates, supporting the management of patients with VTE. Advancements have been made in areas such as the duration of therapy, the choice of dose, early discharge and home management, and the treatment of patients with CAT. Importantly, this year has highlighted that NOACs, such as rivaroxaban, may be able to effectively protect against dangerous recurrent VTE in a broad spectrum of patients and clinical scenarios, to prevent potentially life-threatening events. Rivaroxaban is currently the only NOAC that offers flexible dosing in the extended treatment phase and allows tailored protection according to a patient’s risk profile, without underdosing or compromising on efficacy, to provide the best future for patients with VTE.
References
- Konstantinides SV, et al. Eur Heart J. 2020;41:543–603. Konstantinides SV, et al. Eur Heart J. 2020;41:543–603. Return to content
- Prins MH et al. Risk of recurrent venous thromboembolism according to baseline risk factor profiles. Blood Adv 2018;2:788–796. Prins MH et al. Risk of recurrent venous thromboembolism according to baseline risk factor profiles. Blood Adv 2018;2:788–796. Return to content
- Barco S, Schmidtmann I, Ageno W et al. Early discharge and home treatment of patients with low-risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban: an international multicentre single-arm clinical trial. Eur Heart J 2019: doi:10.1093/eurheartj/ehz367. Return to content
- Young AM, Marshall A, Thirlwall J et al. Comparison of an oral Factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol 2018;36:2017–2023. Return to content
- Raskob G.E., van Es N., Verhamme P. et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615–24. Return to content
- Bledsoe JR, Woller SC, Stevens SM et al. Management of low-risk pulmonary embolism patients without hospitalization: the Low-Risk Pulmonary Embolism prospective management study. Chest 2018;154:249–256. Return to content
- Bayer AG. Xarelto® (rivaroxaban) Summary of Product Characteristics. 2019. Available at: https://www.ema.europa.eu/documents/product-information/xarelto-epar-product-information_en.pdf [accessed 14 November 2019]. Bayer AG. Xarelto® (rivaroxaban) Summary of Product Characteristics. 2019. Available at: https://www.ema.europa.eu/documents/product-information/xarelto-epar-product-information_en.pdf [accessed 14 November 2019]. Return to content
- Konstantinides SV, Torbicki A, Agnelli G et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014;35:3033-3069. Return to content
- Al-Samkari H, Connors JM. The role of direct oral anticoagulants in treatment of cancer-associated thrombosis. Cancers 2018;10:271. Return to content
- Cohen AT, Maraveyas A, Beyer-Westendorf J et al. Patient-reported outcomes associated with switching to rivaroxaban for the treatment of venous thromboembolism in patients with active cancer. European Society of Medical Oncology. Barcelona, Spain, 27 September–1 October 2019, Poster P1774P. Cohen AT, Maraveyas A, Beyer-Westendorf J et al. Patient-reported outcomes associated with switching to rivaroxaban for the treatment of venous thromboembolism in patients with active cancer. European Society of Medical Oncology. Barcelona, Spain, 27 September–1 October 2019, Poster P1774P. Return to content
- Key NS, et al. J Clin Oncol. 2020;38:496–520. Key NS, et al. J Clin Oncol. 2020;38:496–520. Return to content
- National Comprehensive Cancer Network. Cancer-associated venous thromboembolic disease, version 1.2019. Plymouth Meeting, PA, USA: National Comprehensive Cancer Network, Inc. 2019. Available at: https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf [accessed 12 August 2019]. National Comprehensive Cancer Network. Cancer-associated venous thromboembolic disease, version 1.2019. Plymouth Meeting, PA, USA: National Comprehensive Cancer Network, Inc. 2019. Available at: https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf [accessed 12 August 2019]. Return to content
- Farge D, et al. Lancet Oncol. 2019;20:e566–e581. Farge D, et al. Lancet Oncol. 2019;20:e566–e581. Return to content
- Khorana A.A., Noble S., Lee A.Y.Y. et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(9):1891–4. Return to content
- Kearon C and Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:1794–1801. Kearon C and Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood 2014;123:1794–1801. Return to content
- Kearon C., Akl E.A., Ornelas J. et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149(2):315-52. Return to content
- Weitz JI, et al. N Engl J Med. 2017;376:1211–1222. Weitz JI, et al. N Engl J Med. 2017;376:1211–1222. Return to content








